|
|
|
PrologP
Customer Benefits
The program provides an indispensable instrument for predicting logP values before synthesis. This can be found in diversity calculations for combinatorial chemistry, in QSAR, and in situations when the compound displays a logP value which is too low or too high. It is also useful if the compound decomposes during measurement, or when the compound is toxic.
Brief Description
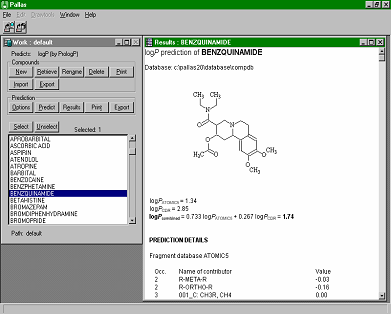 CompuDrug's PrologP calculates the accurate logP values (negative logarithms of the n-octanol/water partition coefficient) for organic compounds, in most cases, within an error range that is not significantly higher than the measurement error of the actual logP value. Three calculation algorithms can perform calculations. Two of them are linear models: one is based on the Rekker fragmental method, the other on the Ghose-Crippen atomic method, while the third one is a neural network model. A further option of the program combines the optimum results obtained by the different models. CompuDrug's PrologP calculates the accurate logP values (negative logarithms of the n-octanol/water partition coefficient) for organic compounds, in most cases, within an error range that is not significantly higher than the measurement error of the actual logP value. Three calculation algorithms can perform calculations. Two of them are linear models: one is based on the Rekker fragmental method, the other on the Ghose-Crippen atomic method, while the third one is a neural network model. A further option of the program combines the optimum results obtained by the different models.
Features at a Glance
-
Predicts the logP value
-
Combines linear and neural network methods
-
Exporting results to any spreadsheet editor (in TAB delimited text file format)
PrologP upgrades
-
Our artificial neural network based approach, using atomic fragmental descriptors, has been developed further to predict the octanol-water partition coefficient (log P) on a wider range of organic compounds. Our present method is based on a trained neural network, but is unique in a way that its input is an extended set of atomic fragmental descriptors.
The developed method has already been published, and the algorithm has been released as part of the version of the Pallas PrologP software.
Our method involves forming the next member of a new class of pseudo-linear algorithms, where the precision of the non-linear approaches is combined with the transparency of earlier linear methods.
The upgrade appears in Pallas for Windows, Pallas for Linux/Unix, Pallas Net, ISIS Plug-in, and Pallas SDK applications.
-
Since the early 1960's, lipophilicity has proven to be very important molecular description, often well-correlated with the bioactivity of chemical entities. Lipophilicity and hydrophobicity are measured by lipophilic and hydrophobic indices, such as the logarithm of a partition coefficient, which reflects the equilibrium partitioning of a molecule between an non-polar and polar (aqueous) phase.
A new artificial neural network using atomic fragmental descriptors has been developed to predict the octanol-water partition coefficient (logP). The fragmental descriptors were obtained from the Atomic2005 linear logP calculation method implemented in Pallas PrologP program. Using a numerically optimized weighted average of the older methods and the current one, the result is significantly more accurate than the previous method, and provides an exceedingly accurate prediction. The new logP prediction method was implemented into the Pallas 3.4 version.
PrologP is operating under the following computing platforms: Windows/Linux, Web application, Software Development Kit
If you need more information about our product, please e-mail CompuDrug International at abc@compudrug.com

|



 CompuDrug's PrologP calculates the accurate logP values (negative logarithms of the n-octanol/water partition coefficient) for organic compounds, in most cases, within an error range that is not significantly higher than the measurement error of the actual logP value. Three calculation algorithms can perform calculations. Two of them are linear models: one is based on the Rekker fragmental method, the other on the Ghose-Crippen atomic method, while the third one is a neural network model. A further option of the program combines the optimum results obtained by the different models.
CompuDrug's PrologP calculates the accurate logP values (negative logarithms of the n-octanol/water partition coefficient) for organic compounds, in most cases, within an error range that is not significantly higher than the measurement error of the actual logP value. Three calculation algorithms can perform calculations. Two of them are linear models: one is based on the Rekker fragmental method, the other on the Ghose-Crippen atomic method, while the third one is a neural network model. A further option of the program combines the optimum results obtained by the different models.
